GREEN ROUTE SYNTHESIS OF COPPER OXIDE (CuO) NANOPARTICLES FOR THE DEGRADATION OF COMMERCIAL DYES USING VICIA FABA LEAF EXTRACT
DOI:
https://doi.org/10.22452/mjs.vol43no3.3Keywords:
nanoparticles, Photocatalytic Degradation, Vicia faba, Methylene blue, rhodamine-BAbstract
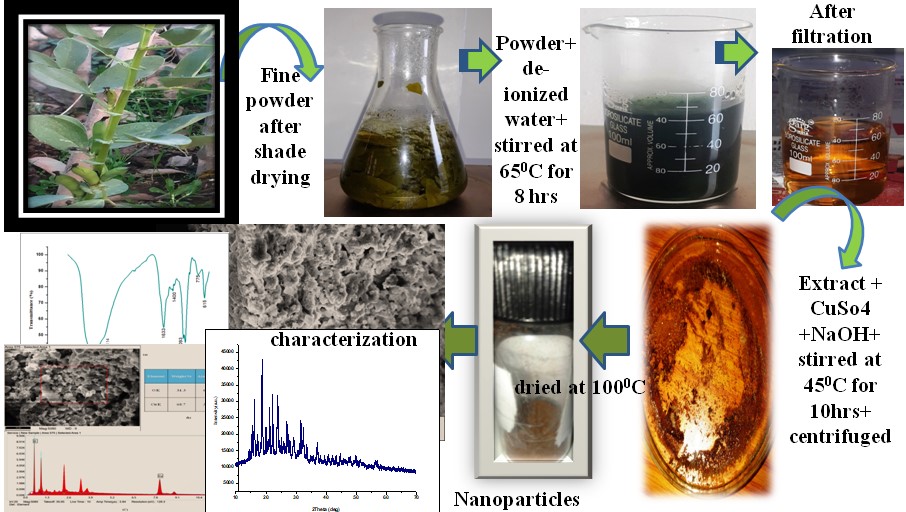
The release of toxic dyes into water effluents by various industries has become a global concern. Therefore, developing novel, straightforward, and economically viable methods or materials for purifying these hazardous pigments is imperative. This study aims to synthesize copper oxide nanoparticles (CuO-NPs) through a green route using Vicia faba leaf extract. UV-Vis spectroscopy reveals an absorption band ranging from 200 nm to 300 nm, with a major absorption peak at 212 nm and an energy band gap of 5.29 eV. FTIR, SEM, EDX, and XRD techniques characterise the synthesised CuO nanoparticles. FTIR analysis identifies functional groups including hydroxyl (OH), aromatic C-H, C=C stretching, carbonyl (C=O), and Cu-O stretching vibrations. Scanning electron microscopy reveals flower-like particles, while EDX analysis confirms the formation of CuO nanoparticles. The XRD pattern indicates a crystalline structure with an average particle size of 27.44 nm. A plot of (αhν)² versus photon energy (hν) was generated to determine the energy band gap, yielding a value of 5.29 eV. Rhodamine-B and methylene blue (MB) dyes were employed to evaluate the photocatalytic degradation of the synthesized CuO-NPs, resulting in correlation coefficients of 0.7154and 0.9702, respectively. Furthermore, the rate constants of these dye reactions were found to be 0.0406min-¹ and0.0343 min-¹.
Downloads
References
Anjum, M., Miandad, R., Waqas, M., Gehany, F., Barakat, M.A. (2016). Remediation of wastewater using various nano-materials. Arab. J. Chem, doi:10.1016/j.arabjc.2016.10.004
Asemani, M., Anarjan, N. (2019). Green synthesis of copper oxide nanoparticles using JUGLANS REGIA leaf extract and assessment of their physico-chemical and biological properties. Green Processing and Synthesis, 8 (1), 557-567. doi.org/10.1515/gps-2019-00252011.
Chen, W., Zheng, L., Jia, R., Wang, N. (2015). Cloning and expression of a new manganese peroxidase from Irpex lacteus F17and its application in decolorization of reactive black 5. Process Biochemistry, 50(11), 1748–1759.
Crisan, M.C., Teodora, M., and Lucian, M. (2022). Copper Nanoparticles: Synthesis and Characterization, Physiology, Toxicity and Antimicrobial Applications. Appl. Sci.., 12(1), 141; https://doi.org/10.3390/app12010141
Dang, T.M.D., Le, T.T.T., Fribourg-Blanc, E., Dang, M.C. (2011). Synthesis and optical properties of copper nanoparticles prepared by a chemical reduction method. Adv Nat Sci: Nanosci Nanotechnol 2(1):015009. doi 10.1088/2043-6262/2/1/015009
Devi, H. S., Singh, T. D. (2014). Synthesis of copper oxide nanoparticles by a novel method and its application in thedegradation of Methyl orange. Advance in Electronic and Electric Engineering, 4(1), 83–88.
Dutta, S., Banerjee, P., Das, P., Mukhopadhyay, A. (2021). Phytogenic synthesis of nanoparticles and their application in photo catalysis of dye rich effluents, Photocatalytic Degradation of Dyes, Elsevier, 647-694
Farré, M. la, Pérez, S., Kantiani, L., Barceló, D. (2008). Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. TrAC-Trends Anal. Chem, 27, 991–1007. doi:10.1016/j.trac.2008.09.010
Jenkins, R., Snyder, R. L. (1996). Introduction to X-Ray Powder Diffractometry, (1st ed). New York, USA, John Wiley and Sons, 554.
Joshi, N.C., Gururani, P., Gairola, S.P. (2022). Metal Oxide Nanoparticles and their Nanocompositebased Materials as Photocatalysts in the Degradation of Dyes. Biointerface Research in Applied Chemistry, 12(5), 6557-6579. doi.org/10.33263/BRIAC125.65576579
Köpke, U., Nemecek, T. (2010). Ecological services of faba bean. Field Crops Research, 115, 217–233. doi.org/10.1016/j.fcr.2009.10.012
Kulkarni, N., and Muddapur, U. (2014). Biosynthesis of Metal Nanoparticles: A Review, Journal of Nanotechnology, Article ID 510246, 8 pages http://dx.doi.org/10.1155/2014/510246
Kumar, M., Mehta, A., Mishra, A., Singh, J., Rawat, M., Basu, S. (2018). Biosynthesis of tin oxide nanoparticles using Psidium Guajava leave extract for photocatalytic dye degradation under sunlight. Mater. Lett, 215, 121–124. doi.org/10.1016/j.matlet.2017.12.07
Mandke, M.V., Pathan, H.M. (2012). Electrochemical growth of copper nanoparticles: structural and optical properties. J Electroanal Chem, 686, 19–24. doi.org/10.1016/j.jelechem.2012.09.004
Muhammad, F.F., Sulaiman, K. (2011). Utilizing a simple and reliable method to investigate the optical functions of small molecular organic flms-Alq3 and Gaq3 as examples. Measurement, 44(8), 1468–1474. doi.org/10.1016/j.measurement.2011.05.017
Mukhlish, M. B., Najnin, F., Rahman, M. M., Uddin, M. J. (2013). Photocatalytic degradation of different dyes using TiO2 withhigh surface area: a kinetic study. Journal of Scientific Research, 5(2), 301–314.
Naveena, D., Dhanabal, R., Bose, A. C. (2022). Investigating the effect of La doped CuO thin film as absorber material for solar cell application.Optical Materials, Volume 127, 112266. doi.org/10.1016/j.optmat.2022.112266
R. Singh, R., Dutta,S. (2018). Synthesis and characterization of solar photoactive TiO2 nanoparticles with enhanced structural and optical properties, Adv. Powder Technol, 29 211–219, doi:http://dx.doi.org/10.1016/j.apt.2017.11.005.
Rajendran, R., Pullani, S., Thavamurugan, S., Radhika, R., Prabha, A.L. (2022). Green Fabrication of silver nanoparticles from Salvia species extracts: characterization and anticancer activities against A549 human lung cancer cell line. Applied Nanoscience, 5(7),1515-1519. https://doi.org/10.1007/s13204-021-02130w.
Sharma, R., Dutta, K. (2015).Studies on the drastic improvement of photocatalytic degradation of acid orange-74 dye by TPPO capped CuO nanoparticles in tandem with suitable electron capturingagents, RSCAdv. 5, 43815–43823, doi:http://dx.doi.org/10.1039/c5ra04179a.
Sharma, V.K., Feng, M. (2017). Water depollution using metal-organic frameworks-catalyzed advanced oxidation processes: A review. J. Hazard. Mater, 372, 3-16. doi:10.1016/j.jhazmat.2017.09.043
Singh, A. K., Bharati, R.C., Manibhushan, N. C., and Pedpati, A. (2013). An assisment of faba bean (Vicia faba L.) current status and future prospect. African Journal of Agricultural Research, Vol. 8(50), pp. 6634-6641, 26 December, do: 10.5897/AJAR2013.7335
Tauc, J., Grigorovici, R., Vancu, A. (1966). Optical properties and electronic structure of amorphous germanium. Physica status solidi (b), 15(2), 627-637. doi.org/10.1002/pssb.19660150224
Valente, I. M., Maia, M.R.G., Malushi, N., Oliveira, H.M., Papa, L., Rodrigues, J.A., Fonseca, A.J.M., Cabrita, A.R.J. (2018). Profiling of phenolic compounds and antioxidant properties of European varieties and cultivars of Vicia faba L. pods. Phytochemistry, 152, 223–229, doi:10.1016/j.phytochem.2018.05.011
Yakuphanoglu, F. Erten, H. (2005). Refractive index dispersion and analysis of the optical constants’ of an ionomer thin flm. Opt Appl, 35(4), 969.
Downloads
Published
How to Cite
Issue
Section
License
Transfer of Copyrights
- In the event of publication of the manuscript entitled [INSERT MANUSCRIPT TITLE AND REF NO.] in the Malaysian Journal of Science, I hereby transfer copyrights of the manuscript title, abstract and contents to the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) for the full legal term of copyright and any renewals thereof throughout the world in any format, and any media for communication.
Conditions of Publication
- I hereby state that this manuscript to be published is an original work, unpublished in any form prior and I have obtained the necessary permission for the reproduction (or am the owner) of any images, illustrations, tables, charts, figures, maps, photographs and other visual materials of whom the copyrights is owned by a third party.
- This manuscript contains no statements that are contradictory to the relevant local and international laws or that infringes on the rights of others.
- I agree to indemnify the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) in the event of any claims that arise in regards to the above conditions and assume full liability on the published manuscript.
Reviewer’s Responsibilities
- Reviewers must treat the manuscripts received for reviewing process as confidential. It must not be shown or discussed with others without the authorization from the editor of MJS.
- Reviewers assigned must not have conflicts of interest with respect to the original work, the authors of the article or the research funding.
- Reviewers should judge or evaluate the manuscripts objective as possible. The feedback from the reviewers should be express clearly with supporting arguments.
- If the assigned reviewer considers themselves not able to complete the review of the manuscript, they must communicate with the editor, so that the manuscript could be sent to another suitable reviewer.
Copyright: Rights of the Author(s)
- Effective 2007, it will become the policy of the Malaysian Journal of Science (published by the Faculty of Science, University of Malaya) to obtain copyrights of all manuscripts published. This is to facilitate:
- Protection against copyright infringement of the manuscript through copyright breaches or piracy.
- Timely handling of reproduction requests from authorized third parties that are addressed directly to the Faculty of Science, University of Malaya.
- As the author, you may publish the fore-mentioned manuscript, whole or any part thereof, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given. You may produce copies of your manuscript, whole or any part thereof, for teaching purposes or to be provided, on individual basis, to fellow researchers.
- You may include the fore-mentioned manuscript, whole or any part thereof, electronically on a secure network at your affiliated institution, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- You may include the fore-mentioned manuscript, whole or any part thereof, on the World Wide Web, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- In the event that your manuscript, whole or any part thereof, has been requested to be reproduced, for any purpose or in any form approved by the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers), you will be informed. It is requested that any changes to your contact details (especially e-mail addresses) are made known.
Copyright: Role and responsibility of the Author(s)
- In the event of the manuscript to be published in the Malaysian Journal of Science contains materials copyrighted to others prior, it is the responsibility of current author(s) to obtain written permission from the copyright owner or owners.
- This written permission should be submitted with the proof-copy of the manuscript to be published in the Malaysian Journal of Science
Licensing Policy
Malaysian Journal of Science is an open-access journal that follows the Creative Commons Attribution-Non-commercial 4.0 International License (CC BY-NC 4.0)
CC BY – NC 4.0: Under this licence, the reusers to distribute, remix, alter, and build upon the content in any media or format for non-commercial purposes only, as long as proper acknowledgement is given to the authors of the original work. Please take the time to read the whole licence agreement (https://creativecommons.org/licenses/by-nc/4.0/legalcode ).





